
Dexcom help center
Do you use an insulin pump or pen with your Dexcom CGM?
Click below to find FAQs for Dexcom CGM and your connected device.
Dexcom G7 training videos
Inserting your Dexcom G7 sensor
Tips and tricks for seamless insertion.
How to get started and set up the Dexcom G7 app
Find out how to use your Dexcom app to start tracking your data.
Dexcom G7 accuracy
Learn more about the accuracy of your Dexcom CGM and the difference between blood glucose monitor and CGM readings.
Dexcom G6 training videos
Inserting your Dexcom G6 sensor and attaching your transmitter
Tips and tricks for a seamless insertion.
How to get started and set up the Dexcom G6 app
Find out how to use your Dexcom app to start tracking your data!
Pairing your transmitter
Learn how to replace your Dexcom transmitter with these simple steps.
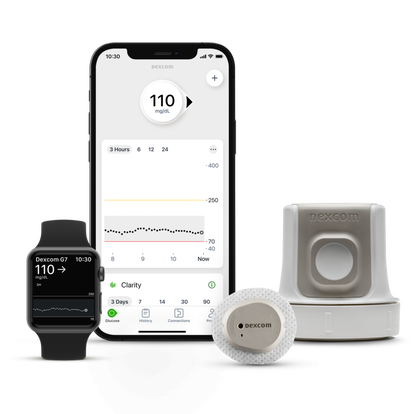
Dexcom G7 Learning Center
Dexcom is here to support you on your journey with us, we’ll show you how to get the best out of your Dexcom G7 and where to find additional help and guidance.

Upgrading to Dexcom G7 is simple if you use Dexcom G6!
Dexcom G7 has all the capabilities you know and love about Dexcom G6, with some amazing new upgrades; we can’t wait for you to experience it.
Click the link below to learn more about upgrading.
Need additional help?

Submit a tech support request
Request Dexcom G6 sensor overpatches

Chat with live tech support
Compatible smart devices sold seprately. For a list of compatible devices, visit dexcom.com/compatibility
BRIEF SAFETY STATEMENT: Failure to use the Dexcom G6 Continuous Glucose Monitoring System (G6) and its components according to the instructions for use provided with your device and available at https://www.dexcom.com/safety-information and to properly consider all indications, contraindications, warnings, precautions, and cautions in those instructions for use may result in you missing a severe hypoglycemia (low blood glucose) or hyperglycemia (high blood glucose) occurrence and/or making a treatment decision that may result in injury. If your glucose alerts and readings from the G6 do not match symptoms, use a blood glucose meter to make diabetes treatment decisions. Seek medical advice and attention when appropriate, including for any medical emergency.
BRIEF SAFETY STATEMENT: Failure to use the Dexcom G7 Continuous Glucose Monitoring System (G7) and its components according to the instructions for use provided with your device and available at https://www.dexcom.com/safety-information and to properly consider all indications, contraindications, warnings, precautions, and cautions in those instructions for use may result in you missing a severe hypoglycemia (low blood glucose) or hyperglycemia (high blood glucose) occurrence and/or making a treatment decision that may result in injury. If your glucose alerts and readings from the G7 do not match symptoms, use a blood glucose meter to make diabetes treatment decisions. Seek medical advice and attention when appropriate, including for any medical emergency.
